 |
|||||||||
| Technology Splitting molecules and how it all comes together |
|||||||||
 |
 |
|
 |
 |
|||||
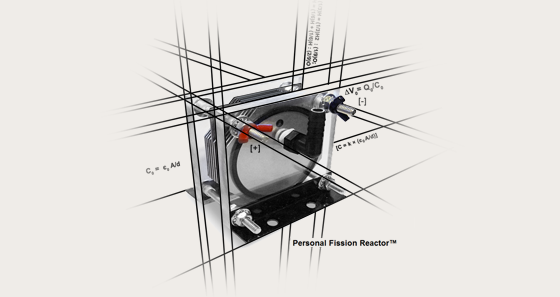
The DR. FISSION™ Personal Fission Reactor™ allows you to create your own fuel, on-demand, from the purest and most abundant source of energy on the planet, water. The Personal Fission Reactor™ splits water, a process known as Molecular Fission™, to safely generate pure hydrogen and oxygen which can be used in a variety of applications. The DR. FISSION™ Personal Fission Reactor™ has numerous in-home applications, including the world's most efficient home and water heating systems. DR. FISSION™ Personal Fission Reactors™ can also power personal, commercial and industrial equipment while saving you thousands by never having to purchase expensive fuels like propane, oxy-acetylene, gasoline or diesel again. The Molecular Fission Reactor™ is a device similar to a capacitor and based on the designs of Faraday and Hofmann's apparatus. Voltage is an electrical potential difference and the potential difference across a parallel plate capacitor without a dielectric has a charge Q0 and capacitance C0. The potential difference across the capacitor is ∆V0= Q0/C0 With a voltmeter the capacitance, the ability to hold a charge, can be measured over time. If a dielectric is placed between the charged plates of a capacitor, the voltmeter displays that the voltage between plates decreases to ∆V. The voltage differences caused by the addition of a dielectric substance are related by a factor of k as follows. ∆V = ∆V0 / k Since ∆V< ∆V0, then k must be greater than 1. k is therefore referred to as the dielectric constant. This constant varies between substances and has been optimized for our uses as Dr. Fission E-Water™ Now, when supplied a current, the charge on the capacitive plates of the Molecular Fission Reactor™ core must change to the following: C= Q0 / ∆V = Q0 / (∆V0 / k) = k × (Q0 / ∆V0) Therefore, C = kC0 So, the capacitance increases by a factor of k when the capacitive reactor is filled with a dielectric. Because, C0 = є0 A/d for parallel plate capacitors. We can then describe the capacitance of a parallel plate capacitor filled with a dielectric as: C = k × (є0A/d) Furthermore, by increasing the electrolytic properties of our water we, in application, reduce the dielectric strength of the water allowing it to decompose into its constituent hydrogen and oxygen under a greatly reduced electric field. This is the core of the mathematical principles that allow the Dr. Fission™ reactors to operate at such high levels of electrical efficiency. From this, we find that capacitance can be altered simply by changing the value of d, the distance between the plates. Since the Dr. Fission™ Personal Fission Reactors™ have been designed to perform in various practical applications, this factor has been optimized to operate at a standard 12 volts. For any given distance, d, the maximum voltage that can be supplied without causing discharge depends on the strength of the dielectric. If the wave, specifically the magnitude, of the electric field in the dielectric exceeds the dielectric strength, the dielectric begins to conduct. What we find with the design of the Dr. Fission™ Personal Fission Reactor™ is that it has the capacity to store high amounts of charge at a relatively low voltage of at or near 12 volts. When a transient analysis is performed on the reactor, the results are quite remarkable. The outcome of this analysis will vary depending on the composition of the electrolyte, specifically its rating of dielectric constant, k. What we find is that, when a voltage of 13.6 volts is applied and then removed, the reactor holds its charge which parabolically decreases over time (up to 18 seconds for the electrolytic mixtures described in this manual). Because of the salinity of our Dr. Fission™ E-Water™, the charged ions in the solution conduct electricity even at times when no voltage is applied, between pulses, by a Dr. Fission™ Pulse Width Modulator circuit. These concepts are what allow us to optimize the electrolysis, or in our case, molecular fission of the water molecule. Gasoline is made of a mixture of hydro-carbons, which are molecules composed of carbon and hydrogen atoms. Typically, in gasoline there are eight carbon atoms along with 18 hydrogen atoms forming each molecule. On average, diesel fuel is 12 carbon atoms and 23 hydrogen atoms per molecule. If you throw a match at gasoline it will burn; diesel fuel won't because it requires compression as well as heat. If full combustion takes place, the products are carbon dioxide and water. This ideal result is not achieved in most vehicle engines. Gasoline burns at about 533°K (260°C, 500°F) in air. Propane by contrast is a molecule of eight hydrogen atoms and three carbon atoms. It burns at around 813°K (540°C, 1004°F). Methane, sometimes called "natural gas," has one carbon atom and four hydrogen atoms per molecule. It burns at about 1923°K (1650°C, 3002°F). These specific figures can vary due to conditions because many factors can change the way these fuels respond. Factors which may cause variation include partial pressure of oxygen, altitude, pressure, humidity and the amount of time required for ignition. However, these figures can be useful when determining hydrogen supply levels for various engines and equipment. The hydrogen you will use comes out of the water moments before it is burned. Once split, the volume ratio between liquid water and HHO, its constituent atoms, is 1800:1. This means that one drop of water (1mL) results in nearly 2 liters of high powered fuel (1800mL). There is no storage tank. There is no pressurized system as with propane. It is all simple and safe. With this technology, the DR. FISSION™ Personal Fission Reactor™ allows you to create your own fuel, on-demand, from the purest and most abundant source of energy on the planet, water. |
|||||||||
|
|
|||||||||
|
|
|||||||||
 |
 |
|
 |
|
|||||
|
|
|
||||||||
 |
|||||||||
 |
 |
||||||||
 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|